 .
.A szövetépítés (tissue engineering) egy viszonylag fiatal, igéretes tudományág. Fő alkalmazási területe az orvostudomány, a nem is annyira távoli jövőben saját sejtjeinkből növeszthetünk szerveket a régiek pótlására. A szövetépítés talán legfontosabb területei az élő és az élettelen anyag közötti interface kialakítása, valamint a sejtek fejlődésének és integrálódásának vezérlése külső jelekkel, jelátvivő molekulákkal. A sejtek fejlődését meghatározó jelek lehetnek fizikaiak, kémiaiak vagy biológiaiak.
 Differenciáltatott C2C12 egér vázizom
sejtvonal mesterséges izom készítéséhez
Differenciáltatott C2C12 egér vázizom
sejtvonal mesterséges izom készítéséhez
My earlier work at University of Central Florida
Peter Molnar, Anupama Natarajan, Ph.D. student, James J. Hickman, Head of Hybrid Neuronal Systems Lab
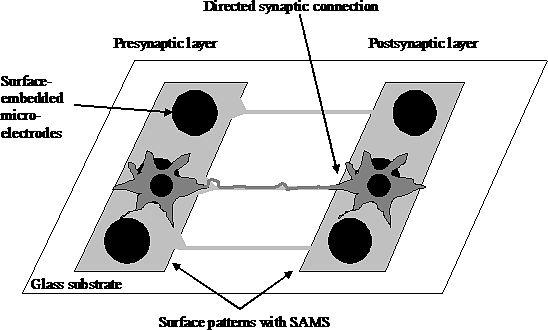
Using functionalized self-assembled monolayers combined with advanced surface patterning methods the inherent differentiation and self-organizing program in the neurons can be controlled and guided to form directed networks. Using the appropriate extracellular clues and cell types, different functional pathways of the brain could be recreated in vitro and used for a better understanding of physiology and pathophysiology of the nervous system. Moreover, surface patterns can be registered with surface-embedded extracellular electrodes allowing chronic or high-throughput recordings of synaptic transmission and network dynamics
 .
.
Original design for a functional high-throughput drug screening method to study drugs acting on synaptic transmission.
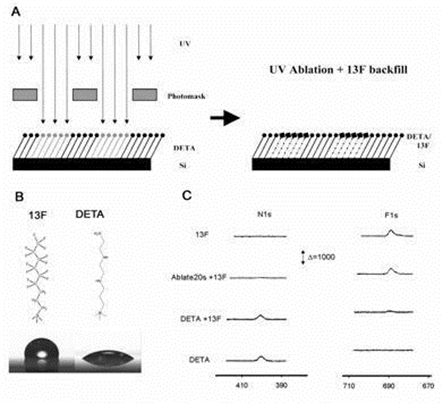
Surface patterns were created using self-assembled monolayers and photolithography. Cell attachment and axonal growth was determined by the surface patterns.
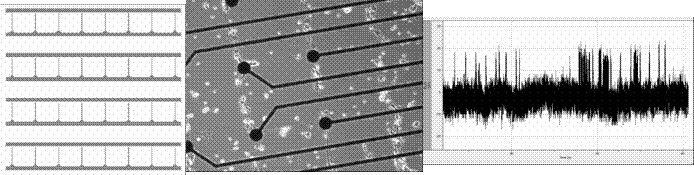
Asymmetric interconnectivity pattern has been designed Patterning of multielectrode arrays has been developed, MEAs can be refunctionalized at least 3 times without degradation of signal quality. As a connected project, we have designed, implemented and characterized two-neuron networks.
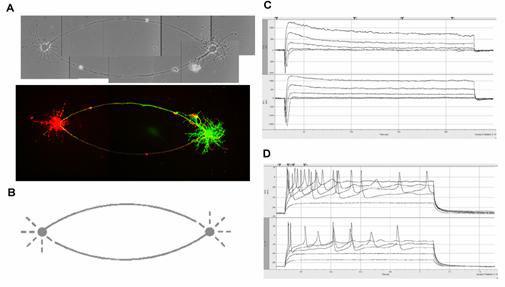
A: Phase contrast and confocal microscopy images of the neurons on the patterns. Cells were filled with a fluorescent dye through the patch pipette. B: Mask design for two-cell networks. The diameter of the soma-attachment area is 25 µm. C: Ionic currents recorded from the patterned cells in voltage-clamp mode. D: Action potentials were evoked by current injection.
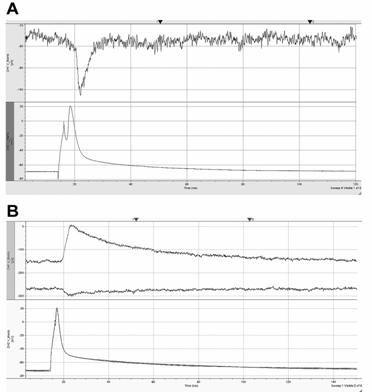
Action potentials were evoked by current injection in one of the cells whereas postsynaptic currents were recorded from the other cell. A: AP - lower trace, EPSC recorded at -70 mV holding – upper trace. B: AP - lower trace, IPSC recorded at -70 and -30 mV holding – upper trace. Note the reversal of the current.
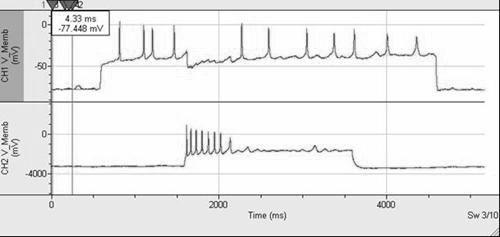
Effect of inhibitory synaptic interaction on the action potential firing of two-cell networks
Also, we are developing a technique to stabilize patterns based on 3D hydrogels